yo? let’s fucking go?
the text of the article:
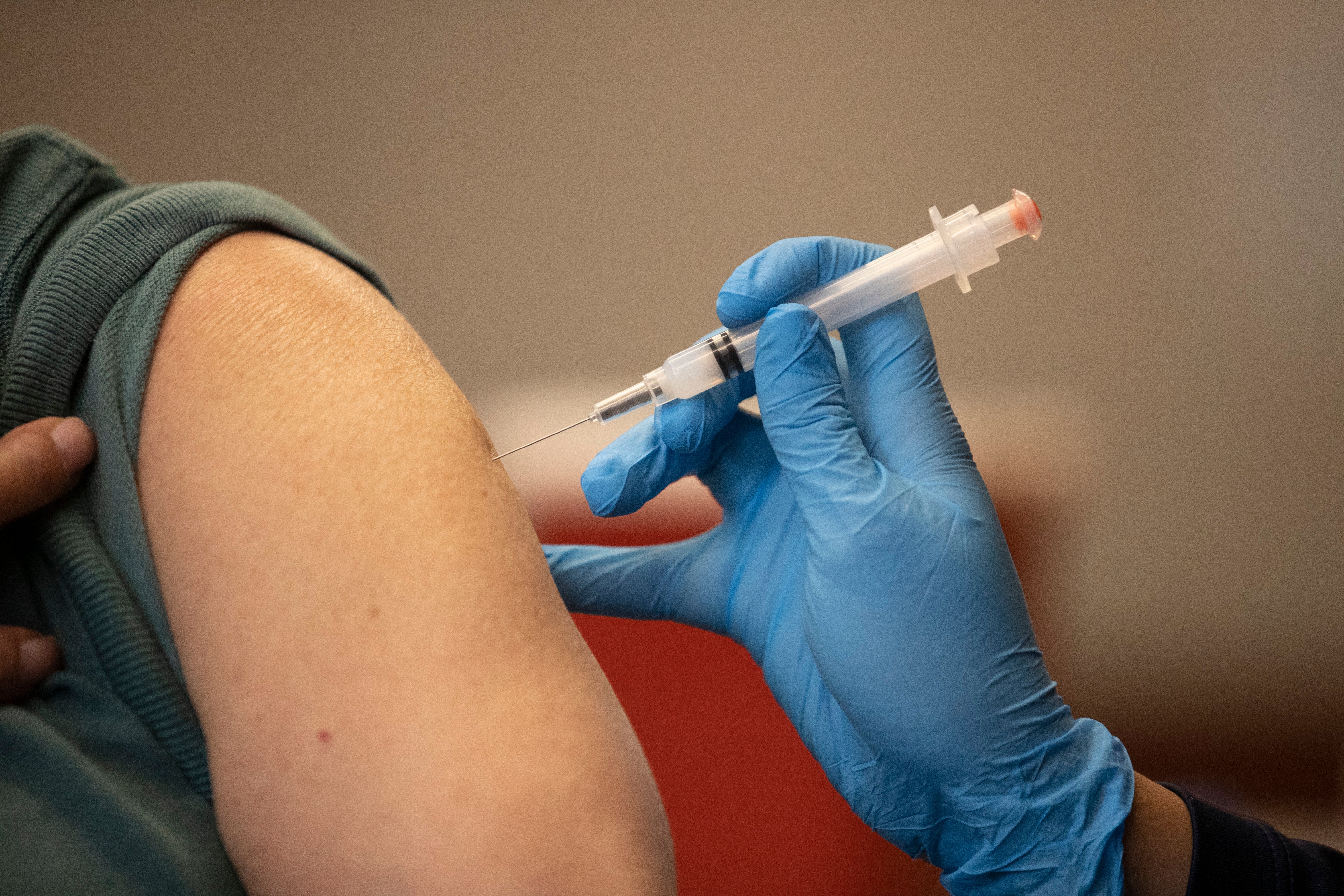
Scientists at UC Riverside have demonstrated a new, RNA-based vaccine strategy that is effective against any strain of a virus and can be used safely even by babies or the immunocompromised.
Every year, researchers try to predict the four influenza strains that are most likely to be prevalent during the upcoming flu season. And every year, people line up to get their updated vaccine, hoping the researchers formulated the shot correctly.
The same is true of COVID vaccines, which have been reformulated to target sub-variants of the most prevalent strains circulating in the U.S.
This new strategy would eliminate the need to create all these different shots, because it targets a part of the viral genome that is common to all strains of a virus. The vaccine, how it works, and a demonstration of its efficacy in mice is described in a paper published today in the Proceedings of the National Academy of Sciences.
“What I want to emphasize about this vaccine strategy is that it is broad,” said UCR virologist and paper author Rong Hai. “It is broadly applicable to any number of viruses, broadly effective against any variant of a virus, and safe for a broad spectrum of people. This could be the universal vaccine that we have been looking for.”
Traditionally, vaccines contain either a dead or modified, live version of a virus. The body’s immune system recognizes a protein in the virus and mounts an immune response. This response produces T-cells that attack the virus and stop it from spreading. It also produces “memory” B-cells that train your immune system to protect you from future attacks.
The new vaccine also uses a live, modified version of a virus. However, it does not rely on the vaccinated body having this traditional immune response or immune active proteins — which is the reason it can be used by babies whose immune systems are underdeveloped, or people suffering from a disease that overtaxes their immune system. Instead, this relies on small, silencing RNA molecules.
“A host — a person, a mouse, anyone infected— will produce small interfering RNAs as an immune response to viral infection. These RNAi then knock down the virus,” said Shouwei Ding, distinguished professor of microbiology at UCR, and lead paper author.
The reason viruses successfully cause disease is because they produce proteins that block a host’s RNAi response. “If we make a mutant virus that cannot produce the protein to suppress our RNAi, we can weaken the virus. It can replicate to some level, but then loses the battle to the host RNAi response,” Ding said. “A virus weakened in this way can be used as a vaccine for boosting our RNAi immune system.”
When the researchers tested this strategy with a mouse virus called Nodamura, they did it with mutant mice lacking T and B cells. With one vaccine injection, they found the mice were protected from a lethal dose of the unmodified virus for at least 90 days. Note that some studies show nine mouse days are roughly equivalent to one human year.
There are few vaccines suitable for use in babies younger than six months old. However, even newborn mice produce small RNAi molecules, which is why the vaccine protected them as well. UC Riverside has now been issued a US patent on this RNAi vaccine technology.
In 2013, the same research team published a paper showing that flu infections also induce us to produce RNAi molecules. “That’s why our next step is to use this same concept to generate a flu vaccine, so infants can be protected. If we are successful, they’ll no longer have to depend on their mothers’ antibodies,” Ding said.
Their flu vaccine will also likely be delivered in the form of a spray, as many people have an aversion to needles. “Respiratory infections move through the nose, so a spray might be an easier delivery system,” Hai said.
Additionally, the researchers say there is little chance of a virus mutating to avoid this vaccination strategy. “Viruses may mutate in regions not targeted by traditional vaccines. However, we are targeting their whole genome with thousands of small RNAs. They cannot escape this,” Hai said.
Ultimately, the researchers believe they can ‘cut and paste’ this strategy to make a one-and-done vaccine for any number of viruses.
“There are several well-known human pathogens; dengue, SARS, COVID. They all have similar viral functions,” Ding said. “This should be applicable to these viruses in an easy transfer of knowledge.


i’m a molecular biologist and would love to see the actual paper (because i think this article is horribly written and really doesn’t explain this very well), but there exists research already for this concept. hypothetically, it would be extremely difficult for a virus to mutate to block siRNAs as they target entire essential genes, such as structural ones or essential replication proteins, so the virus would have to fundamentally change many different and important genes to evade siRNA detection, and also because in therapeutic settings, many different siRNAs targeting different sites would be used simultaneously. even in viruses that have evolved to have RNAi suppressing proteins, some research shows that synthetic siRNAs can still have an effect.
I found this as well,
https://academic.oup.com/nar/article/50/1/333/6470682
If even a small amount cells are infected, couldn’t a cascade effect still trigger and overpower what this vaccine is trying to do? That said, it could definitely reduce the chance of initial infection, right?
I’ve been out of school long enough that some of this is going over my head. But yeah if this is applicable to any virus it is absolutely game changing.
yes, i believe the intention here (as is outlined in the paper you linked) is to target viruses before they infect a cell for the greatest chance of preventing what you described? but again, its really unclear to me without the original paper but that link in the article isn’t working which really sucks.
And I guess by reducing initial infection you’re still reducing the chance any given cell is infected, lowering viral load, symptoms, chance to mutate etc.
Yeah I need a crash course on this, sometimes it feels like medicine advances so fast it’s hard to keep up.
You should comment and volunteer to help mod /c/healthcare! https://hexbear.net/post/2239683
it definitely is going extremely fast lol, i feel like every week there’s something new and wild and it’s hard to keep up with.
i’ll definitely leave a comment! i’m not a healthcare provider at all and specialize more in microorganisms but i guess all life has a similar foundation, so id love to contribute.
Yep, most modern drugs wouldn’t exist if we didn’t understand microbiology
deleted by creator
thanks for finding the paper and summarizing it! yeah i can definitely see how this wouldn’t really be as effective as they touted, especially with the vector problem. though there are a lot of different viruses that could be used as vectors right? i knew someone who did research into parvoviruses and it seems like they could work too. i still think it’s a cool idea though and i’m hoping it finds more applications.