Summary
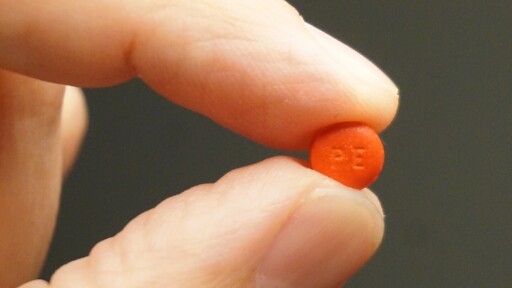
The FDA has proposed phasing out oral phenylephrine, a common decongestant in cold medicines like Sudafed PE and DayQuil, after studies showed it is no more effective than a placebo.
The drug, ineffective when swallowed due to breakdown in the stomach, remains usable in nasal sprays.
Alternatives include pseudoephedrine, nasal sprays, and steroid treatments like Flonase.
The regulatory process to remove phenylephrine could take over a year, but experts argue removing ineffective options will help consumers choose better remedies for congestion. Drugmakers are expected to challenge the proposal.


Not the person you’re replying to and have nothing to add to this specific conversation, but I just wanted to commend you on this style of communication. It was so clear and level and I see no way anyone (in good faith) could find anything to misconstrue from your statements. This is som 10/10 internet communication and I will be studying the way you laid this comment out for future use.